ELogBook Implementation
Profile
One of the leading pharma API company, engaged mainly in the export and regulated by USFDA guidelines. It has 3 plants in India and 1 overseas.
Value Delivered
Project Scope
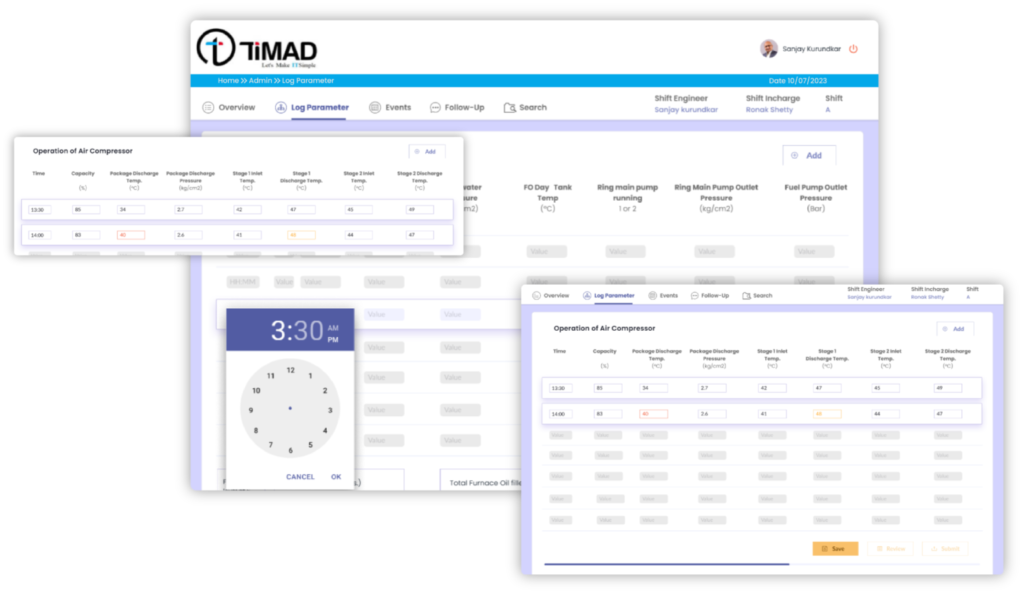
- Digitized and imported all the existing logbook templates for QA and QC Department
- Configured Review and Approval process of the shift logbooks
- Auto emailing of the submitted logbooks to key stakeholders
- Provision to record the critical events and Follow-ups in the shift with auto forward facility
Benefits
- All logbooks of the respective department are 100% digitized with 100% elimination of paper based process.
- On screen data range alerts helped minimize the erroneous manual entries
- Controlled user access and audit trail helped in achieving better compliance for regulatory audits
- Digitized data is available for future analytics.
