ELogBook Implementation
Profile
One of the MNC pharma mainly into formulation and USFDA certified
Value Delivered
Project Scope
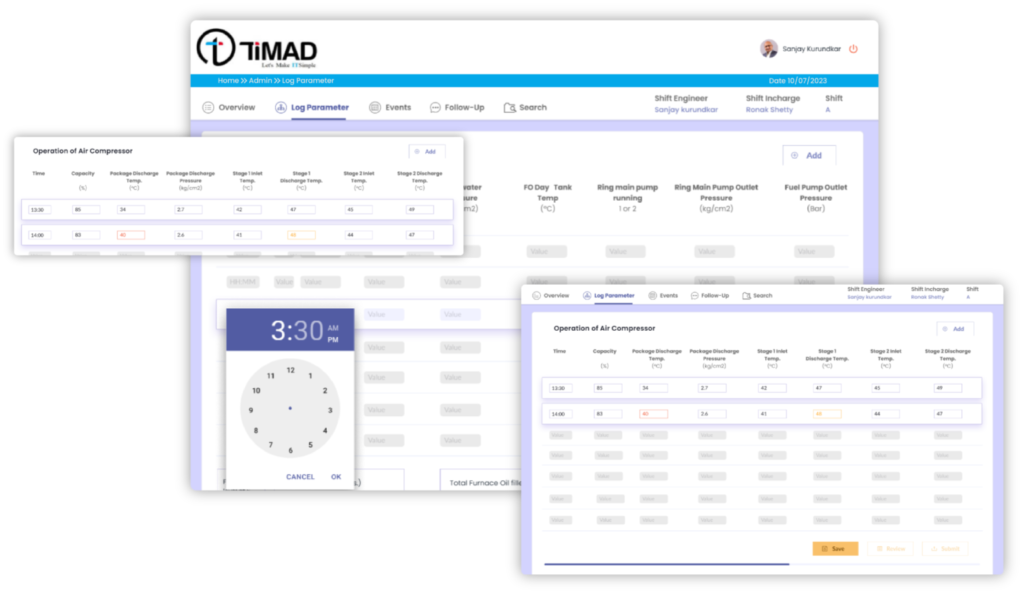
- Digitized and imported all the existing logbook templates for QA and QC Department
- Configured Review and Approval process of the shift logbooks
- Auto emailing of the submitted logbooks to key stakeholders
- Provision to record the critical events and Follow-ups in the shift with auto forward facility
- Auto computation of the Pass/Fail results based on the Acceptance Criteria
Benefits
- All logbooks of the respective department are 100% digitized with 100% elimination of paper based process.
- On screen data range alerts helped minimize the erroneous manual entries
- Developed analytical reports to monitor the equipment performance based on the log entry data
- On screen Maker/Checker arrangement with provision to add review remarks
